Propane, C3H8, natural gas, CH4, and phosphorous, P4 react with oxygen O2, and these reactions release energy in the form of heat and light. No doubt, according to the principle of conservation of energy, energy is required to reverse the reactions. Thus, energy stored in chemicals (compounds) and energy released or absorbed in chemical reactions are called chemical energy, which also covers topics such as bond energy, ionization potential, electron affinity, electronegativity, lattice energy, etc.
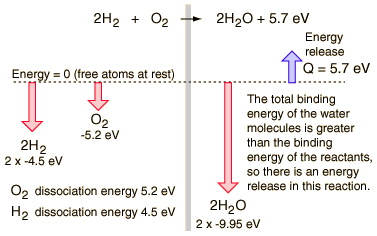
For example, at standard conditions, the combustion of 1.0 mole hydrogen with oxygen releases 285.8 kJ of energy. We represent the reaction.
H2(g) + 1/2 O2 -> H2O (l), dH = -285.8 kJ/mol
where dH represent the heat (or enthalpy) of reaction, and a negative value means that the heat is released. Usually, dH is represented by DH in textbooks, but using dH notation is much less work on Internet documents.
For the reverse reaction, 285.8 kJ/mol is required, and the sign for dH value changes.
H2O (l) -> H2(g) + 1/2 O2, dH = +285.8 kJ/mol
A Chemical Energy Level Diagram
------------H2(g) + 1/2O2
|
| |
286 kJ | | -286 kJ
| |
| ¯
------------ H2O
We can also use an energy level diagram to show the relative content of energy. The energy content of H2(g) + 0.5 O2 is 285.8 kJ higher than a mole of water, H2O.
Oil, gas, and food are often called energy by the news media, but more precisely they are sources of (chemical) energy -- energy stored in chemicals with a potential to be released in a chemical reaction. The released energy performs work or causes physical and chemical changes.
It is obvious that the amount of energy released in a chemical reaction is related to the amount of reactants. For example, when the amount is doubled, so is the amount of energy released.
2 H2(g) + O2 -> 2 H2O (l), dH = -571.6 kJ/mol
Example 1 shows the calculation when the amount of reactants is only a fraction of a mole.
Example 1
How much energy is release when a balloon containing 0.15 mole of hydrogen is ignited in the air?
Solution
The amount released is 0.15 mol * 285.8 kJ/mol = 42.9 kJ
Discussion
The sudden release of energy causes an explosion.
Endothermic and Exothermic Reactions
A reaction that releases energy is called exothermic reaction. Energy is released in the form of heat, light and (pressure-volume) work. For example, when methane or propane is oxidized by O2, the heat released causes the gas to expand (explosion in some cases); releasing heat & light and doing work at the same time. In this case, the energy source came from chemical reactions instead of at the expense of internal energy described in the previous module.
Endothermic reactions absorb energy, and in all cases, the energy is supplied from another source, in the form of electrical energy, heat or light.
Pressure-volume Work in Chemical Reactions
Many chemical reactions involve gases, and when a gas is formed, it displaces other gases by pushing them out against a pressure. Work, defined in Newtonian physics as a force times the distance along the force direction, is performed in such an action. The work is called the pressure-volume (P-V) work, which is a form of energy and it must be analyzed and its quantity included in chemical energy calculations.
The SI units for pressure are N m-2 and that of the volume is m3. Pressure times volume gives the unit of N m, which is the definition of joule,
1 Pa * 1 m3 = 1 N m-2 m
= 1 N m
= 1 J
Since 1 atm = 101300 Pa, and 1 L = 0.001 m3. Thus,
1 atm L = 101.3 J.
The P-V work under constant pressure (P) is simply the pressure times the change in volume dV.
w = - P dV
This method applies to reactions that produce gases, which are released into the atmosphere. When work is done by the system, the work is negative, as the formula indicates. In reactions where gases are consumed to produce liquid or solid, work is done on the system by its environment. The work is positive.
In cases the pressure varies, an integral approach is required to evaluate the pressure volume work.
w = - Ó d (P V)
= - Ó P dV - (the integral of) V dP
The negative sign is retained, because the work done by the system is negative. However, the integral of P V work depends on the path, and we will not get into the detail discussion at this stage.
Example 2
In the reaction to produce oxygen,
KClO3(s) = KCl(s) + 3/2 O2(g),
calculate the pressure-volume work done by 8.2 g of KClO3.
Solution
The molar mass of KClO3 is 123.5 g/mol and 8.2 g is 0.067 mol. Thus, the amount of oxygen produced is 0.10 (= 0.067*2/3) mol. Apply the ideal gas law to the pressure volume work (P V), w have
P V = n R T
w = - D P V
= - D n R T
= - 0.10 mol*8.312 (J / (mol K))*298 K
= - 248 J
Discussion
The work done is due to the formation of gas O2 which expands against the atmosphere of 1.0 atm or 101.3 kPa. The volume changes of the solids are insignificant compared to that of the gas.
In case both pressure and volume change, and the work is the difference of the pressure-volume product, DP V.
Enthalpy
The enthalpy, usually represented by H is the energy released in a chemical reaction under constant pressure, H = qP. The enthalpy is a convenient property to evaluate for reactions taking place at constant pressure. Enthalpy differ from internal energy, E, defined in Energy as the energy input to a system at constant volume. The energy released in a chemical reaction raises the internal energy, E, and does work under constant pressure at the expense of energy stored in compounds. Thus,
H = qP = E + P dV
Of course, the enthalpy change (dH) of a chemical reaction depends on the amount of reactants, the temperature, and pressure. Under normal conditions, the ideal gas law can be applied to give reasonable results.
Like the internal energy, enthalpy is also a thermodynamic state function, depending only on the initial and final states of the system, but not on the rate of reaction.
Standard Enthalpy of Reaction
In order to make the data useful for scientific and engineering applications, there is a general agreement to report and tabulate enthalpy changes for a mole of reaction at a standard temperature and pressure. Such quantities are called the standard enthalpy of reaction.
In handbooks and textbooks, the standard enthalpy change is represented by Ho. For simplicity, we use dHo to represent the changes of standard enthalpy in our discussion to avoid (very) slow loading of the delta onto your computer.
Example 3
The standard enthalpy for the combustion of methane is 890.4 kJ per mole,
CH4(g) + 2 O2(g) -> CO2(g) + 2 H2O(g), dHo = -890.4 kJ/mol
calculate the standard enthalpy change when 1.0 cubic meter of natural gas is burned converting to gaseous products.
Solution
When 1.0 mol or 22.4 L of CH4, at 273K and 1 atm, is oxidized completely, the standard enthalpy change is 890.4 kJ. One cubic meter is 1000 L (/22.4 = 44.6 mol). Thus, the standard enthalpy of change is,
dH = 44.6 mol * 890.4 kJ/mol = 39712 kJ or 39 million joules.
A problem can be made up using any of the following standard enthalpy of reactions. These are given here to illustrate the type of reactions and the representation of enthalpy of reactions.
2 H(g) -> H2(g) dHo = -436 kJ/mol
2 O(g) -> O2(g) dHo = -498 kJ/mol
H2O(l) -> H2O(g) dHo = 44 kJ/mol at 298 K
H2O(l) -> H2O(g) dH = 41 kJ/mol at 373 K, non-standard condition
Mg(s) + S(s) -> MgS(s) dHo = -598 kJ/mol
2 H(g) + O(g) -> H2O(g) dHo = -847 kJ/mol
Cu(s) + 1/2O2(g) -> CuO(s) dHo = -157 kJ/mol
1/2N2(g) + O2(g) -> NO2(g) dHo = 34 kJ/mol
Mg(s) + 1/2O2(g) -> MgO(s) dHo = -602 kJ/mol
2 P(s) + 3 Cl2(g) -> 2 PCl3(s) dHo = -640 kJ/mol
2 P(s) + 5 Cl2(g) -> 2 PCl5(s) dHo = -880 kJ/mol
C(graphite) + 2 O(g) -> CO2(g) dHo = -643 kJ/mol
C(graphite) + O2(g) -> CO2(g) dHo = -394 kJ/mol
C(graphite) + 2 H2(g) -> CH4(g) dHo = -75 kJ/mol
2 Al(s) + Fe2O3(s) -> Al2O3(s) + 2Fe(s) dHo = -850 kJ/mol
As we shall see, the application of Hess Law will make these data very useful. For example, applying Hess law using a few of these reactions enable us to calculate the heat of combustion of methane to form liquid water (as opposed to gaseous water) and carbon dioxide,
CH4 + 2 O2 -> 2 H2O(l) + CO2(g) dH = -980 kJ/mol.
Enthalpy is an important topic in thermodynamics. Various methods have been devised for the accurate measurement of heat of reaction under constant pressure or under constant volume. This link gives a more advanced treatment on enthalpy.
Standard Enthalpy of Formation, dHf
When the standard enthalpy is for a reaction that forms a compound from its basic elements also at the standard state, the standard enthalpy of reaction is called the standard enthalpy of formation, represented by dHof. Unless specified, the temperature is 298 K.
Table of dHof
Compound dHof
MgS -598 kJ/mol
CuO -157
PCl3 -320
PCl5 -440
H2O -286
NO2 + 34
MgO -602
CO2 -394
CH4 -75
In the above list, some of the equations lead to the formation of a compound from its elements at their standard state. These equations and their enthalpy of formation are given below:
Mg(s) + S(s) -> MgS(s) dHof = -598 kJ/mol
P(s) + 3/2 Cl2(g) -> PCl3(g) dHof = -320 kJ/mol
P(s) + 5/2 Cl2(g) -> PCl5(g) dHof = - 440 kJ/mol
H2(g) + 1/2 O2(g) -> H2O(g) dHof = -286 kJ/mol
1/2N2(g) + O2(g) -> NO2(g) dHof = + 34 kJ/mol
Cu(s) + 1/2 O2(g) -> CuO(s) dHof = -157 kJ/mol
Mg(s) + 1/2 O2(g) -> MgO(s) dHof = -602 kJ/mol
C(graphite) + O2(g) -> CO2(g) dHof = -394 kJ/mol
C(graphite) + 4 H2(g) -> CH4(g) dHof = -75 kJ/mol
In all the above equations of reaction, the right hand side has only one product and that its coefficient is 1. A general rule is to consider standard enthalpy of formation of all elements at the standard condition to be zero. Then, there is no need to write the complete equation for the tabulation of the standard enthalpy of formation. The above list can be simplified to give the table shown here.
A simple application of the standard enthalpy of formation is illustrated by Example 4.
Example 4
For NH3, dHf = -46.1 kJ/mol. Estimate energy released when 10 g of N2 react with excess of H2 to form ammonia.
Solution
Ten grams of nitrogen is less than 1 mol, and we carry out the calculation in the following manner:
1 mol N2 - 46.1 kJ
10 g N2 ---------- ---------- = - 32.9 kJ
28.1 g N2 0.5 mol N2
Thus, 32.9 kJ is released when 10 g of N2 is consumed.
Standard enthalpies of formation and standard entropies are important thermodynamic data, and this link gives an extensive table of values for some key compounds.
The principle of conservation of energy leads to the formulation of the Hess law. It's application makes the enthalpy of reaction and standard enthalpy of formation very useful.
Confidence Building Questions
*
Which one of the following will you buy it for its (potential) chemical energy: gasoline, beer, diamond, dry ice, a music CD, or a chemistry text book?
Skill:
Identify compounds that provide energy.
A bottle of beer also provides a bit of energy, but the energy aspect is insignificant.
*
Which of the following processes is endothermic?
a. condensing steam into water
b. burning a candle
c. melting ice cream
d. cooling hot coffee
e. formation of snow flakes
Skill:
Identify process as endothermic or exothermic reaction.
*
Which one of the following compounds when formed from its elements at the standard condition is endothermic?
a. CuO
b. PCl3
c. PCl5
d. H2O
e. NO2
Discussion:
The common sense tells us that the formation of a though d are exothermic. Check the standard enthalpy of formation. A lot of energy is required to break the triple bond in 0.5 N2 + O2 -> NO2
*
A 10.0-L tire contained air at 3.0 atm exploded on a free way. How much energy is available for the explosion?
Skill:
To evaluate pressure-volume work of a system. The final volume should be 30.0 L at 1.0 atm, a net gain of 20.0 L in volume.
*
Calculate the pressure volume work for the reaction:
ZnO(s) = Zn(s) + 0.5 O2
Skill:
Apply the ideal gas law to evaluate the P V work.
*
In a chemical reaction, an unknown amount of propane is burned. It released 500000 kJ for the BBQ, and performed 9999 kJ of pressure work. Which one can you calculate from the information given above?
a. internal energy change
b. standard enthalpy change
c. molar enthalpy change of CO2
d. enthalpy of formation of propane
e. enthalpy of reaction
Skill:
Define enthalpy of reaction as the energy release in a reaction and the work done by the system.
*
The enthalpy of formation is as indicated:
H2(g) + 0.5 O2 -> H2O(l), dHf = -285.8 kJ/mol
Calculate the enthalpy of reaction when 1 mole of O2 reacts with 4 moles of H2 at the standard conditions.
Skill:
Calculate the energy change using standard enthalpy of reaction and standard enthalpy of formation.
*
The enthalpy of formation is as indicated:
H2(g) + 0.5 O2 -> H2O(l), dHf = -285.8 kJ/mol
Calculate the enthalpy required to decompose 1 mole of water by electrolysis, all products and reactant at their standard conditions.
Skill:
Calculate the energy change using standard enthalpy of formation.
*
From the following data
P(s) + 3/2 Cl2(g) -> PCl3(g) dHof = -320 kJ/mol
P(s) + 5/2 Cl2(g) -> PCl5(g) dHof = - 440 kJ/mol
Evaluate the standard enthalpy of the reaction:
PCl3(g) + Cl2(g) -> PCl5(g) dHof = ? kJ/mol
Skill:
Apply the principle of conservation of energy. Solving this problem is a prelude to Hess law.
*
A problem to be defined.
Skill:
cchieh@sciborg.uwaterloo.ca