Consider the combination of two molecules of H2 with one molecule of O2 to form two molecules of water, H2O. Energetically, the process can be considered to require the energy to dissociate the H2 and O2, but then the bonding of the H2O returns the system to a bound state with negative potential. It is actually more negative than the bound states of the reactants, and the formation of the two water molecules actually releases 5.7 electron volts of energy .
The balance of energy before and after the reaction can be illustrated schematically with the state in which all atoms are free taken as the reference for energy.
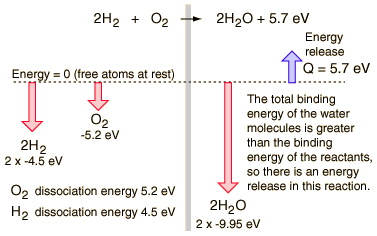
If the dissociation energies of the H2 and O2 and the energy release upon their reaction were measured, that would offer an experimental path to determining the total bond energy of the H2O molecule.
An energy balance approach can also be useful in the analysis of an ionic bond.
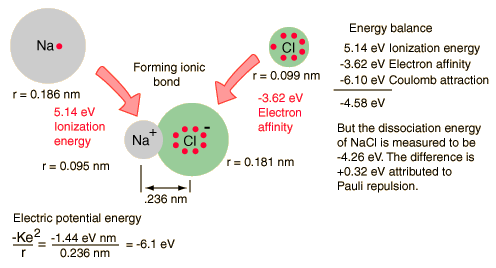
Available experimental parameters for sodium, chlorine and the NaCl molecule provide data for calculation of the dissociation energy of the molecule from conservation of energy. The steps toward forming the NaCl ionic molecule could be seen as (1) providing the ionization energy of 5.14 eV to ionize Na, then (2) the recovery of -3.62 eV from the electron affinity of Cl, then the binding energy of the electric attractive forces based on the known bond length of 0.236 nm. But this process does not match the measured dissociation energy of NaCl, 4.26 eV. This reveals the presence of another repulsive energy term called Pauli repulsion (+0.32 eV).

No comments:
Post a Comment